Pediatric Medicine 2018
Details of Pediatric Medicine 2018 Conference in USA:
ConferenceSeries LLC Ltd is privileged to announce its “International Conference on Pediatric Hospital Medicine” with the innovative theme “Dynamic and Collegial approach of Pediatric Hospitalists” which will be held during August 29-30, 2018 inBoston, USA.
We cordially welcome all the eminent researchers, students and delegates to take part in this upcoming Pediatric Hospital Medicine conference to witness invaluable scientific discussions and contribute to the future innovations in the field of Pediatrics.
Pediatric Medicine 2018 conference will focus on the latest and exciting innovations and providing direct care to children with a wide range of medical. The conference includes Child health care workshops, symposiums, special keynote sessions conducted by eminent and renowned speakers who excel in the field of Pediatric Medicine which include the topics clinical conundrums, pediatric and hospital medicine specialties, challenges in pediatric drug development, pediatric drug delivery and dosage forms, hospitalists: categorization, pediatric hospitalist responsibilities, hospital acquired conditions, pediatric nosocomial infections, pediatric nosocomial infections: diagnosis and treatments, pediatric hospitalization process improvement techniques, pediatric nursing and care, pediatric herbal medicine etc. This International Pediatrics Conference also encourages the active participation of young student researchers as we are hosting Poster Award Competition and Young research Forum at the conference venue.
Conference Series LLC Ltd is organizing Pediatric Medicine Conference in 2018 at Boston, USA. We organize Pediatric Medicine Meetings in the fields related to Pediatric and hospital medicine specialties, Challenges in Pediatric drug development, Hospitalists: Categorization, Pediatric Hospitalist responsibilities and Hospital acquired conditions.
Sessions/Tracks
Track 1: Clinical conundrums
Pediatric clinical conundrums are some interesting topics of discussion for clinicians around the world. More than theoretical approach a real time case study or case report is more effective to deal with critical situations arising in the hospital. This session will focus on all such case studies and real experiences of paediatricians and other physicians in the hospital. A systematic approach towards better pediatric healthcare will be the main aim of this conference session.
Track 2: Pediatric and hospital medicine specialties
The American Academy of Pediatrics(AAP) started the section on Hospital Medicine with approximately just over 400 members in the year 2005 and presently with the rise in importance of this speciality now has over 2,000 members. With the sole outlook and motive of transitioning patients safely back into the community at the time of their discharge is the aim of every hospitalist. The inpatient teams consisting several sub-specialities and including the patient, family and social and other services coordinate to form an interdisciplinary plan of healthcare that is necessary.This session of the conference will aim to associate with such care givers who are instrumental in providing the best and required treatments to the patients.
Track 3: Challenges in Pediatric drug development
Pediatric drug development is a challenge for over the past half a century. There are quite a few childhood diseases which have significant occurrence rates in pediatric subjects but for which there are no approved drugs. Reports say that as a result of low prioritization of pediatric drug development globally about 75% of drugs do not carry regulatory approval for use in infants or children; ~66% of medications used in children in the US are used off label, 50% in Europe and in Japan it is less than 20%. Most medications in these regions donot contain package inserts have adequate information for treating infants or children. This session will address all such issues including R&D, F&D and regulatory framework with research scientists from different pharmaceutical industries and also drug discovery researchers.
Track 4: Pediatric drug delivery and dosage forms
A different or modified drug delivery system is required by the pediatric patients compared to other subsets of the population due to their continuing development hence dosing and administration requirements. Conventional formulations are not designed for pediatric patients. Hence it becomes necessity to manipulate and compound the medication accordingly and this has become common practice. The pharmacokinetic and pharmacodynamic profile of a drug varies broadly depending on the factors like developmental stage of a child, necessitating dose flexibility to suit the dosing requirements etc. Excipients which are commonly regarded as safe may pose a safety risk for child group in addition to other considerations of the formulation and development of the dosage form a drug. Palatability and ease of swallowing are also considered as pivotal parametrs for the acceptability of medicines intended for children, who due to their age possess distinct preferences and swallowing abilities than other subsets of the population. New formulation design approaches like multiparticulate drug delivery systems, orodispersible tablets (ODTs), orodispersible films (ODFs), and chewable formulations are gainig importance based on their respective efficacy, PK/PD factors and patient compliances.
Track 5: Hospitalists: Categorization
From the time of inception hospitalists have emerged as a fast growing group of physicians in the United States. Reports suggest that in almost 30% hospitals, wherein 55% hospitals consist beds equal to 200 or more, have hospitalists as their medical staffs. Presently, more than 12,000 hospitalists are practicing in the United States. With time if this hospitalist model continues to have expansion at the present rate, the number of hospitalists will eventually rise to 30,000 in the United States by 2019. With its success rate appreciably rising hospitalist programs are adopted in several countries outside North America, including Australia, New Zealand, Argentina, Brazil, Chile, Columbia, Spain, Sweden, and Singapore.
Track 6: Pediatric Hospitalist responsibilities
Most hospitalists’ time are engaged in delivering and coordinating the healthcare for a wide range of conditions affecting hospitalized children. Reports say that patients under the care of hospitalists have decreased duration of stay and lesser associated costs when compared to physicians with principally outpatient responsibilities. The range of services provided by a pediatric hospitalist group is by large dependent on the requirement of the situation and the institution. Also the ability of the group to provide these services is an important factor, which may include the following:
Ø Consultation or short-stay observation unit services
Ø Coordinated, family-centered interdisciplinary admission and discharge planning that includes the essential role of the primary care physician
Ø Delivery room services
Ø General inpatient pediatric care
Ø Newborn nursery care
Ø NICU and PICU coverage
Ø Palliative care
Ø Pediatric emergency department evaluation
Ø Perioperative surgical and medical subspecialty care4
Ø Sedation services
Track 7: Hospital acquired conditions
In the year 2011 The Centers for Medicare and Medicaid Services (CMS) have finalized rules that prohibit Medicaid payments for the additional cost of services which result from the preventable healthcare acquired illnesses or injuries, that are generally referred to provider-preventable conditions (PPCs). Basically, there are two distinct categories of PPCs:
1. Health Care-Acquired Conditions (HCACs)- pertaining to Medicaid inpatient hospital settings
2. Other Provider-Preventable Conditions (OPPCs)- applying to Medicaid inpatient and outpatient health care settings.
It may include conditions like:
I. A foreign object retained within a patient´s body after surgery.
II. The development of an air embolism within a patient´s body.
III. A patient blood transfusion with incompatible blood.
IV. A patient´s development of stage III or stage IV pressure ulcers.
V. Patient injuries resulting from accidental falls and other trauma
VI. A patient´s manifestations of poor glycaemic control
VII. A patient´s development of a catheter-associated urinary tract infection.
VIII. A patient´s development of a vascular catheter-associated infection.
IX. A patient´s development of a surgical site infection
X. Surgery or other invasive procedure on the wrong patient.
XI. Wrong surgery or other invasive procedure on a patient.
XII. Surgery or invasive procedure performed on the wrong site.
Track 8: Pediatric Nosocomial infections
Nosocomial infections (NI) are one of the most important causes of morbidity and mortality in pediatric hospitals. Nosocomial (hospital-acquired) infections cause a number of major complications which potent to serious illnesses. Severely ill patients pose a greater risk of acquiring nosocomial infection. This in turn implies to the problem being greatest in intensive care units. However, studies have demonstrated that nosocomial infections are largely preventable. Most Hospital-acquired infections are caused by viral, bacterial, and fungal pathogens. Most common types are bloodstream infection (BSI), pneumonia (eg, ventilator-associated pneumonia [VAP]), urinary tract infection (UTI), and surgical site infection (SSI).
Track 9: Pediatric infectious diseases : diagnosis and treatments
For diagnosis of nosocomial infections the Laboratory investigations should be additionally guided by the results of detailed physical examination and clinical manifestations. Interpreting laboratory results is critical for nosocomial infections as not all bacterial or fungal growth on a culture are pathogenic. Growth on cultures may often reflect simple microbial colonization. Considering the points like *clinical presentation of the patient *reason for obtaining the test *process by which the specimen was obtained *presence of other supporting evidence of infection is critical.
Track 10: Pediatric hospitalization process improvement techniques
Discharge from the hospital for most pediatric patients implies that the child is clinically improving, but all the same it also creates potential risk of the transition of care. In a study, almost 49% of patients had more than 1 medication error at discharge, which potent to increase their likelihood for readmission. In other studies, approximately 10% to 20% of patients were found to have an adverse event after discharge, with about half of these events deemed to be preventable. It is because of the potential for errors and variability in the discharge process, that recently the Children’s Hospital Association (CHA) formed the first pediatric improvement collaborative to examine whether the shared improvement strategies would affect discharge-related care failures, parent-reported readiness for discharge, and readmission.
Track 11: Pediatric Nursing and Care
Children—they aren't just miniature adults. Children's perceptions of pediatric nursing care have not been systematically taken into account in the development of the quality of care. Their bodies function differently, absorb medications differently, and process thoughts differently. Starting a normal healthcare regimen early in life enables children to become healthy adults. Nurses, no matter where they work, come into contact with children. It's important to know that children require special handling. Developing trust is key when trying to gain the pediatric patient's cooperation. Understanding the differences in children is important to care for them safely. The family should be included in the child's care, and parent concerns should be taken seriously. Parents can be a valuable tool in assessing and caring for pediatric patients. With the correct knowledge and attitude, treating children and their families can be a wonderful experience.
Market Analysis
The global market for pediatric medicine was around $83.6 billion in 2014, potentially rising by about 4% annually until 2019 reaching $100.7 billion. Among the booming market rise categories Vaccines, inflammatory diseases, respiratory conditions and viral infections are key therapy areas, in addition to central nervous system (CNS) treatments and oncology. Obesity, conjointly with diabetes in later life, is a special concern (as well as for Type I diabetes itself, generally a childhood condition). Moreover, the growth of specialty drugs under the Orphan Drug Act has been an added boon for pediatric medicine with children being the most preys to rare diseases. Orphan drug diseases have also provided new opportunities for the industry to target pediatric drugs to treat childhood cancers, metabolic diseases and immune-related diseases. Pediatric medicine market’s significant rise through 2019 can be attributed to the industry becoming more familiar with designing and implementing trials in the pediatric population. In addition, the focus on commercially viable targets of high unmet clinical needs such as diseases designated under the Orphan Drug Act (ODA), those for pediatric oncology and the treatment of metabolic disorders. However, surveys say that when assessing for clinicians prescribing a medicine which is off-label to a pediatric patient, 20% of physicians don’t inform the parent or guardian that they are doing so, and only 24% of physicians require legal consent. Thus it is evident that there is an increased risk of malpractice lawsuits without informed consent.
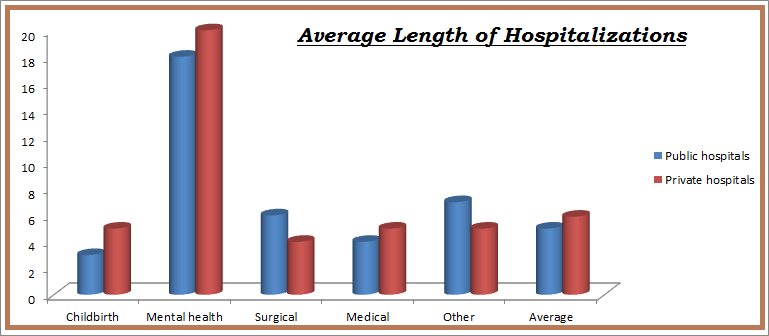
Reports say that researchers who have studied the impact of hospital medicine in 12 U.S. cities have found a “stunning” level of variation. The researchers were surprised at different models hospitalist programs carried out around the country. To their surprise different types of hospitalist programs function side by side in single markets. Nevertheless, when it comes to utilizing hospitalists, the American Hospital Association reported that 60 percent of hospitals staff these specialists today, up from only 30 percent 10 years ago. Physician/provider staffing, recruitment, and training are the major cost components for PHM programs. Overhead is generally low and includes such items as office space, office supplies, and telecommunication/internet expenses. Clinical supplies are provided by the hospital; all the hospitalist needs is a stethoscope, otoscope, and ophthalmoscope. Many hospitals and health systems have made a substantial investment in the acquisition and employment of physicians, even though many still lose $100,000 to $175,000 a year per physician. The hospital market in US is almost around $1tr with a steady growth of 3% every year from 2012-2017.
Visa Application
For conference attendance and participation only Business Visa should be applied. Contact your nearest travel agent/visa information center/US Embassy for the correct application form.
All visas for visiting US shall be processed by respective authorities only upon submission of proper documents through proper channel.
In case of non-furnishing of documents, non-adherence to guidelines visas shall be cancelled by respective authorities.
The minimum supportive documents that might be required while applying for US visa include:
Letter of invitation,
Abstract acceptance letter (if speaker),
Registration payment receipt,
Accommodation confirmation letter issued under conference letter head.
For letter of invitation and accommodation confirmation, payment of registration fees and accommodation charges is a pre-requisite.
Mandate documents required from conference secretariat should be obtained only through Rachael Roy
For more details please contact
Rachael Roy
Program Manager
Pediatric Pharma 2018
E: pediatricpharma@conferencesamerica.org
Past Conference Report
Pediatrics 2017
Conference Series LLC Ltd hosted the “10th Annual World Congress on Pediatrics, Pediatric Gastroenterology and Nutrition”, during March 23-25, 2017 at Holiday Inn Orlando Airport, USA, with the theme, “Innovative Schemes and Research Strategies to Prevent Pediatric Diseases”, which was a great success. Eminent keynote speakers from various reputed institutions and organizations addressed the gathering with their resplendent presence.
We extend our grateful thanks to all the momentous speakers, conference attendees who contributed towards the successful run of the conference.
Pediatrics and Pediatric Gastroenterology 2017 witnessed an amalgamation of peerless speakers who enlightened the crowd with their knowledge and confabulated on various latest and exciting innovations in all areas of pediatrics research.
Pediatrics and Pediatric Gastroenterology 2017 Organizing Committee extends its gratitude and congratulates the Honorable Moderators of the conference, Dr. Naveed Durrani, McMaster Children Hospital, Canada, Joseph L Mathew, Postgraduate Institute of Medical Education and Research, India and Dr. Ganeswara Rao Melam, King Saud University, Saudi Arabia for their remarkable contribution towards smooth functioning of this esteemed event.
Conference Series LLC Ltd extends its warm gratitude to all the Honorable Guests and Keynote Speakers of Pediatrics and Pediatric Gastroenterology 2017:
-
Dr. Steven J Melnick, Nicklaus Children’s Hospital, USA
-
Dr. Rodrigo Vianna, Miami Transplant Institute-University of Miami, USA
-
Dr. Michael J Wilsey, Johns Hopkins All Children’s Hospital, USA
-
Dr. Yasser K Rashed, Menoufiya University, Egypt
-
Dr. Geir Ogrim, Ostfold Hospital Trust, Norway
Poster Evaluation Committee and the Winner:
Pediatrics and Pediatric Gastroenterology 2017 would like to acknowledge Dr. Neelam Mohan for her evaluation of the poster session and we are glad to congratulate Dr. Judy Prehn, Director of Clinical Education and Associate Professor, William Carey University, USA for receiving the Best Poster Award of the conference.
Conference Series LLC Ltd is privileged to felicitate Pediatrics and Pediatric Gastroenterology 2017 Organizing Committee, Keynote Speakers, Chairs & Co-Chairs and also the Moderators of the conference whose support and efforts made the conference to move on the path of success. Conference Series LLC Ltd thanks every individual participant for the enormous exquisite response. This inspires us to continue organizing events and conferences for further research in the field of Pediatrics.
Conference Series LLC Ltd therefore is glad to announce its “11th Annual World Congress on Pediatrics”, which will be held during March, 2017 in New York, USA (pediatrics.conferenceseries.com). We cordially welcome all the eminent researchers, students and delegates to take part in this upcoming conference to witness invaluable scientific discussions and contribute to the future innovations in the field of Pediatrics with 20% abatement on the Early Bird Prices. The first round of Abstract submission deadline is July 27, 2017.
Bookmark your dates for “Pediatrics 2018” as the Nominations for Best Poster Awards and Young Researcher Awards are open across the world.
Testimonials of Experts on Pediatrics and Pediatric Gastroenterology 2017, March 23-25, Orlando, USA:
-
I would like to thank you for inviting me as a speaker. It was a true honor, and I thoroughly enjoyed it.
Dr. Shridevi Pandya Shah, Rutgers-NJMS, USA
-
It was an excellent conference with lots of learning opportunities, Multi-specialty topics really helped a lot.
Dr. Naveed Durrani, McMaster Children Hospital, Canada
-
It was a good opportunity to share the information about each interest.
Dr. Go Ichikawa, Nasu Red Cross Hospital, Japan
-
I really appreciate your support on my attendance of the excellent meeting, 10th Annual World Congress on Pediatrics 2017. Traveling and staying Orlando was quite comfortable and the discussion with world top researchers inspired me.
Dr. Kumiko Taira, Tokyo Women’s Medical University Medical Center East, Japan
-
The World Congress on Paediatrics 2017 at Orlando was an academically stimulating event. It brought together specialists from diverse backgrounds and varying interests. The scientific sessions were thought-provoking and interesting. Clinical and research experiences in different disciplines were shared
Dr. Joseph L. Mathew, P.G.I.M.E.R, India
Past Reports Gallery










